When I was a kid, I really, really wanted a chemistry set. One year for Christmas I got an electronics kit that I really tried to like, but it didn’t thrill me. Then I received a geology kit for my birthday. I wasn’t overly interested in the rocks it contained, but there was a serious looking hammer and some test tubes of mysterious liquids that were like a mini chemistry set and were therefore Quite Exciting. They didn’t explode or smoke or anything dramatic when mixed, but I pretended to know what I was doing, and I’m doing it still. Pretending that is. I took chemistry all through high school, and it turns out, it’s not really all that exciting. Or not exciting enough for me to actually remember any of it anyway (in common with the rest of my high school education). But all of that is entirely irrelevant to the issue at hand: high ph in my aquaponics system.
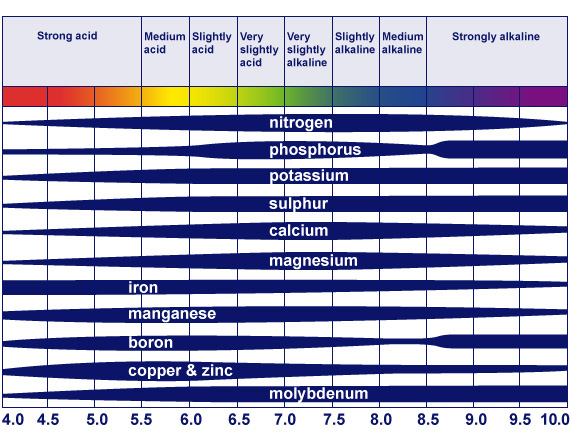
Ph is a measure of acidity or alkalinity. A ph of 7 is considered neutral, while lower values are acidic and higher are alkaline. The scale is logarithmic, so a difference of one on the scale represents a ten-fold increase or decrease in acidity. The water in my system is about 8.3, which is very alkaline. Why this matters is that plants are limited in their ability to absorb nutrients by the ph of their environment. The chart below shows the relative amounts of different nutrients that are absorbed at different ph levels. My water is at the “medium alkaline” level and as you can see this affects phosphorous, iron, boron, copper and zinc.
 So what to do? Lowering the ph is difficult and risky, because the fish are going to be negatively affected by any change of more than 0.2 in a day. The carbonates in the water that cause the ph to be high, also buffer the ph so that it remains stable. It is difficult to change the ph by a little – you keep adding acid with no apparent effect, then the ph crashes and fish die. Luckily, the natural nitrifying process actually consumes carbonates, slowly but steadily. What I have begun to do is to treat the top up water with hydrochloric acid (also known as muriatic acid) in a bucket for a day or so before I add it to the system. I drop the ph of the water in the bucket to 6, which consumes carbonates. When I add the water, slowly over the next day, it doesn’t change the ph of the system noticeably but neither does it contribute additional carbonates to the system. This means that the carbonates in the water will actually decrease through nitrification instead of being constantly replenished. It may take some months before my ph is in the perfect range, but it will have happened safely.
So what to do? Lowering the ph is difficult and risky, because the fish are going to be negatively affected by any change of more than 0.2 in a day. The carbonates in the water that cause the ph to be high, also buffer the ph so that it remains stable. It is difficult to change the ph by a little – you keep adding acid with no apparent effect, then the ph crashes and fish die. Luckily, the natural nitrifying process actually consumes carbonates, slowly but steadily. What I have begun to do is to treat the top up water with hydrochloric acid (also known as muriatic acid) in a bucket for a day or so before I add it to the system. I drop the ph of the water in the bucket to 6, which consumes carbonates. When I add the water, slowly over the next day, it doesn’t change the ph of the system noticeably but neither does it contribute additional carbonates to the system. This means that the carbonates in the water will actually decrease through nitrification instead of being constantly replenished. It may take some months before my ph is in the perfect range, but it will have happened safely.
In the meantime, I need to be concerned with iron. Fish feed contains some iron, but the fish require it for themselves, and there is very little excess and not enough for plants which are struggling to absorb any at all because of high ph. Many of my plants were showing signs of iron deficiency (yellow leaves) so after consulting the handy internets, I purchased some garden-type chelated iron. I’ll probably need to add a half teaspoon of iron every month or so until I see no more signs of deficiency. I think some aquapons add iron throughout the life of their systems.


You might want to be careful with this. It can bind up oxygen and kill your fish. Also, unless it’s EDDHA it’s unlikely to be plant-available in significant quantities at the pH of a typical aquaponics system.